The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water.A solution at 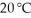 that is 0.401 M in MnSO4 monohydrate is best described as a(n) ________ solution.The formula weight of MnSO4 monohydrate is 168.97 g/mol.
that is 0.401 M in MnSO4 monohydrate is best described as a(n) ________ solution.The formula weight of MnSO4 monohydrate is 168.97 g/mol.
Definitions:
Online Sales Training
A form of instruction or education delivered over the internet that aims to improve the skills and knowledge of individuals in selling products or services electronically.
On-the-Job Training
The training received by employees during the course of their current employment to enhance skills and performance.
Selling Skills
Abilities necessary to effectively persuade customers to purchase goods or services, including communication, negotiation, and closing techniques.
Selling
The process of persuading a customer to purchase a product or service.
Q12: A 0.133 mol sample of gas in
Q23: A mixture of He and Ne at
Q37: The mechanism for formation of the product
Q43: If pressure and temperature are kept constant,the
Q47: Which of the following substances is more
Q67: The substance with the largest heat of
Q78: It was determined experimentally that the reaction
Q82: Which reaction will shift to the right
Q90: Which one of the following gases would
Q129: Under constant conditions,the half-life of a first-order