The molarity of urea (MW = 60.0 g/mol) in a solution prepared by dissolving 11 g of urea in 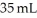 of H2O is ________ M.Assume the volume of the solution does not change when dissolving the urea.
of H2O is ________ M.Assume the volume of the solution does not change when dissolving the urea.
Definitions:
Antiaromatic
A compound that is cyclic, conjugated, but does not follow Huckel's rule, often being less stable due to its electron configuration.
Nonaromatic
Organic compounds that do not fulfill the criteria for aromaticity, lacking the special stability observed in aromatic systems.
Fused-ring Heterocycle
A cyclic compound where two or more rings share two or more common atoms, with at least one of the rings containing atoms other than carbon.
Purine
A heterocyclic aromatic organic compound, forming the basis of two of the four nucleotides that constitute DNA and RNA (adenine and guanine).
Q34: All of the following are a type
Q59: Which of the following aqueous solutions will
Q61: 0.7515 moles of nitrogen gas and 0.1135
Q66: The initial discovery of a liquid crystal
Q83: The empirical formula of an addition polymer
Q87: Given the following reaction at equilibrium,if K<sub>c</sub>
Q105: The property responsible for the "beading up"
Q117: A sample of H<sub>2</sub> gas (12.28 g)occupies
Q135: What is the formula weight of iron(III)chloride
Q169: At at 315 K and 1.16 atm,the