A solution containing 10.0 g of an unknown liquid and 90.0 g water has a freezing point of -3.33 °C.Given 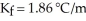 for water,the molar mass of the unknown liquid is
for water,the molar mass of the unknown liquid is 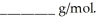
Definitions:
Federal Government
The central government of a federation that has specific powers over national concerns, while sharing other powers with subnational jurisdictions.
Q7: Which of the following gases would have
Q19: If a reaction is endothermic,_ the reaction
Q20: Calculate the concentration (in M)of hydroxide ions
Q23: An unknown metal crystallizes in a primitive
Q25: Which one of the following pairs cannot
Q38: An acid containing the COOH group is
Q96: At elevated temperatures,methylisonitrile (CH<sub>3</sub>NC)isomerizes to acetonitrile (CH<sub>3</sub>CN):
Q102: Of the acids in the table below,_
Q106: How many moles of an unknown gas
Q131: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The of