Determine the freezing point (°C) of a 0.015 molal aqueous solution of MgSO4.Assume i = 2.0 for MgSO4.The molal freezing-point-depression constant of water is 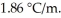
Definitions:
Apoplast
A continuum consisting of the interconnected, porous plant cell walls, along which water moves freely. Compare with symplast.
Plasmodesmata
Microscopic channels that traverse the cell walls of plant cells and some algal cells, enabling transport and communication between them.
Symplast
The interconnected cytoplasmic network in plants, allowing for transport and communication between cells via plasmodesmata.
Primary Eudicot
A subgroup of eudicots, plants that are part of a larger group of flowering plants characterized by seeds with two embryonic leaves or cotyledons.
Q2: Of the following,_ is a valid statement
Q11: Which compound has the strongest intermolecular forces?<br>A)CCl<sub>4</sub><br>B)CI<sub>4</sub><br>C)CH<sub>4</sub><br>D)H<sub>2</sub><br>E)O<sub>2</sub>
Q18: What is the conjugate acid of OH<sup>-</sup>?<br>A)O<sub>2</sub><br>B)H<sub>2</sub>O<br>C)O<sup>-</sup><br>D)O<sup>2-</sup><br>E)H<sub>3</sub>O<sup>+</sup>
Q20: A 25.0 mL sample of a solution
Q46: What is the predominant intermolecular force in
Q79: Pairs of liquids that will mix in
Q113: The root-mean-square speed of CO at 113
Q114: 10.0 grams of argon and 20.0 grams
Q119: Of the following gases,_ will have the
Q131: When solutions of strong electrolytes in water