The freezing point of ethanol ( 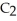
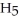 OH) is -114.6 °C.The molal freezing point depression constant for ethanol is 2.00 °C/m.What is the freezing point (°C) of a solution prepared by dissolving 50.0 g of glycerin
OH) is -114.6 °C.The molal freezing point depression constant for ethanol is 2.00 °C/m.What is the freezing point (°C) of a solution prepared by dissolving 50.0 g of glycerin 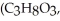 a nonelectrolyte) in 200.0 g of ethanol?
a nonelectrolyte) in 200.0 g of ethanol?
Definitions:
Glocalization
The process of adapting a product or service to suit local tastes and cultural preferences while maintaining a global outlook.
Mega Phone 4
A fictional or specific product name, possibly referring to a model of a phone or a communication device.
State Control
The extent to which a government exercises authority and regulatory powers over economic and social policies within its territory.
Global Communication Strategy
A coordinated approach to managing and delivering a company's message to its international audience, ensuring consistency across diverse markets.
Q43: Of the following,_ is an exothermic process.<br>A)melting<br>B)subliming<br>C)freezing<br>D)boiling<br>E)All
Q50: The primary source of the specificity of
Q57: What is the predominant intermolecular force in
Q65: A sample of gas (1.9 mol)is in
Q80: An ideal gas differs from a real
Q82: Which reaction will shift to the right
Q99: All of the following are characteristics of
Q122: The boiling points of normal hydrocarbons are
Q151: The concentration of urea (MW = 60.0
Q172: A sample of oxygen gas (O<sub>2</sub>)was found