Calculate the freezing point of a 0.05500 m aqueous solution of NaN 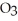 .The molal freezing-point-depression constant of water is
.The molal freezing-point-depression constant of water is 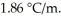 (Assume 100% ionization of NaNO3.)
(Assume 100% ionization of NaNO3.)
Definitions:
Base Rate
In statistics and diagnostic testing, the prevalence or general rate of occurrence of a condition, characteristic, or event within a given population.
Millon
A psychological assessment tool named after Theodore Millon, used for personality disorders and psychopathology.
Standardized Score
A score that has been transformed to fit a normal distribution, allowing for comparison across different tests or populations.
Myers-Briggs
A personality inventory that categorizes individuals into 16 distinct personality types based on preferences in how they perceive the world and make decisions.
Q6: With what compound will NH<sub>3</sub> experience only
Q22: A sample of H<sub>2</sub> gas (5.0 mol)effused
Q36: Which monomer is polymerized to make natural
Q41: _ liquid crystals are colored because the
Q43: KF crystallizes in a face-centered cubic cell.What
Q54: Which solution has the greatest buffering capacity?<br>A)0.335M
Q61: The equilibrium constant for the gas phase
Q100: A flask contains a mixture of N<sub>2</sub>
Q115: The concentration of iodide ions in a
Q115: CO (5.00 g)and CO<sub>2</sub> (5.00 g)were placed