Calculate the freezing point of a solution containing 20 grams of KCl and 2200.0 grams of water.The molal-freezing-point-depression constant ( 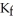 ) for water is
) for water is 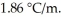 (Assume 100% ionization of KCl.)
(Assume 100% ionization of KCl.)
Definitions:
Abilities
Skills or competencies that enable an individual to perform specific tasks or activities effectively.
Positive Views
The adoption of an optimistic perspective, focusing on the good aspects of a situation rather than the negative.
Performance Comparison
The act of evaluating and contrasting the outcomes or abilities of individuals or systems against others or against a set of standards.
Coordinate Senses
The ability to process and integrate information from different sensory channels into a coherent perception.
Q3: Rates of reaction can be positive or
Q6: If a rate law is first order
Q40: In an exothermic equilibrium reaction,decreasing the reaction
Q50: What is the [ <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="What
Q56: The relationship between the concentrations of reactants
Q60: In which aqueous system is <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q61: What two compounds react to form the
Q64: What volume (mL)of fluorine gas is required
Q83: If pressure and temperature are kept constant,the
Q89: Which statements about viscosity are true?<br>(i)Viscosity increases