The decomposition of 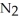
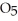 in solution in carbon tetrachloride proceeds via the reaction 2N2O5 (soln) → 4NO2 (soln) + O2 (soln)
in solution in carbon tetrachloride proceeds via the reaction 2N2O5 (soln) → 4NO2 (soln) + O2 (soln)
The reaction is first order and has a rate constant of 4.82 × 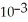
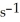 at 64 °C.If the reaction is initiated with 0.072 mol in a 1.00-L vessel,how many moles remain after 151 s?
at 64 °C.If the reaction is initiated with 0.072 mol in a 1.00-L vessel,how many moles remain after 151 s?
Definitions:
GPA
Grade Point Average, a numerical calculation that represents the average value of the accumulated final grades earned over time from courses taken.
Line Graph
A type of graph that uses lines to connect individual data points, displaying trends over a period of time or category.
Bar Chart
A graphical representation of data using bars of different heights or lengths to compare values across different categories.
Histogram
A graphical representation of the distribution of numerical data, showing the frequency of data intervals.
Q4: The average pH of the oceans is
Q21: Under ordinary conditions,a substance will sublime rather
Q30: What is the conjugate acid of NH<sub>2</sub><sup>-</sup>?<br>A)NH<sub>2</sub><sup>+</sup><br>B)NH<sub>3</sub><sup>+</sup><br>C)NH<sub>4</sub><sup>+</sup><br>D)NH<sub>3</sub><br>E)NH<sub>4</sub>OH
Q42: What is the mass % of ammonium
Q62: What is the concentration (M)of [A] after
Q67: The concentration of Na in seawater is
Q81: An aqueous solution at 25.0°C contains [
Q83: How many moles of B are present
Q90: The rate law of the overall reaction
Q106: An acetic acid aqueous solution contains 22%