The half-life of a first-order reaction is 13 min.If the initial concentration of reactant is 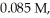 it takes
it takes  for it to decrease to 0.055 M.
for it to decrease to 0.055 M.
Definitions:
Random Error
Variability in data that is caused by unpredictable and unavoidable fluctuations in the measurement process.
Linearly Correlated
A statistical relationship between two variables in which changes in one variable are associated with proportional changes in another variable along a straight line.
Normally Distributed
Describes a symmetric, bell-shaped distribution of data where the mean, median, and mode are equal, prevalent in many natural phenomena.
Coefficient of Determination
A statistical measure that explains the proportion of the variance for a dependent variable that's explained by an independent variable or variables in a regression model.
Q18: What is the conjugate acid of OH<sup>-</sup>?<br>A)O<sub>2</sub><br>B)H<sub>2</sub>O<br>C)O<sup>-</sup><br>D)O<sup>2-</sup><br>E)H<sub>3</sub>O<sup>+</sup>
Q31: The acid-dissociation constant at 25.0 °C for
Q31: What is the molality of a 10.0%
Q34: A 25.0 mL sample of an HCl
Q45: CsCl crystallizes in a unit cell that
Q48: What is the pH of a 0.40
Q52: The equilibrium expression for K<sub>p</sub> for the
Q55: What value is represented by the y-intercept
Q67: What is the mole fraction of hydrochloric
Q149: A solution is prepared by dissolving 24.7