The decomposition of 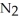
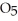 in solution in carbon tetrachloride proceeds via the reaction 2N2O5 (soln) → 4NO2 (soln) + O2 (soln)
in solution in carbon tetrachloride proceeds via the reaction 2N2O5 (soln) → 4NO2 (soln) + O2 (soln)
The reaction is first order and has a rate constant of 4.82 × 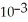
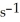 at 64 °C.If the reaction is initiated with 0.072 mol in a 1.00-L vessel,how many moles remain after 151 s?
at 64 °C.If the reaction is initiated with 0.072 mol in a 1.00-L vessel,how many moles remain after 151 s?
Definitions:
Pension Plans
Financial programs that provide income to individuals during retirement, funded by employers, employees, or both.
Full-Time Employees
Individuals who are contracted to work a minimum number of hours specified by their employer, typically 30 to 40 hours a week.
Healthcare Spending Accounts
A savings account that allows employees to set aside pre-tax dollars to pay for eligible healthcare expenses.
Employee Benefits
Various types of non-wage compensation provided to employees in addition to their normal wages or salaries.
Q5: According to the phase diagram shown above,what
Q14: Natural,unpolluted rainwater is typically acidic.What is the
Q41: Consider the following chemical reaction: CO (g)+
Q53: An aqueous solution of ammonia at 25.0
Q63: Components of Air Mole Fraction Nitrogen 0.781<br>Oxygen
Q83: The value of K<sub>eq</sub> for the following
Q98: What is the molal concentration of a
Q113: A buffer solution contains 0.100 M fluoride
Q124: Which of the following aqueous solutions has
Q130: Which one of the following statements regarding