Consider the following reaction at equilibrium: 2NH3 (g)  N2 (g) + 3H2 (g)
N2 (g) + 3H2 (g)
Le Châtelier's principle predicts that the moles of 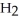 in the reaction container will increase with ________.
in the reaction container will increase with ________.
Definitions:
Burnout
A state of physical, emotional, and mental exhaustion caused by prolonged stress, often resulting from overwork or workplace frustration.
Idealistic
Characterized by the pursuit or belief in high and often unrealistic goals or principles.
Extraverted
A personality trait characterized by being outgoing, talkative, and energized by interactions with other people.
HDL Cholesterol
High-density lipoprotein cholesterol, often referred to as "good" cholesterol, which helps reduce the risk of heart disease.
Q6: Using the data in the table,which of
Q14: At 400 K,the equilibrium constant for the
Q17: What is the percent gold in a
Q75: What is the order of the reaction
Q90: Determine the pH of a 0.35 M
Q94: The equilibrium expression for K<sub>p</sub> for the
Q109: The pH of a solution prepared by
Q111: The entropy of the universe is _.<br>A)constant<br>B)continually
Q117: The overall order of a reaction is
Q120: The principal component of smog is sulfur