Multiple Choice
Dinitrogen tetroxide partially decomposes according to the following equilibrium: 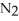
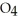 (g) →
(g) → 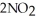 (g) A 1.000-L flask is charged with 9.20 × 10-3 mol of
(g) A 1.000-L flask is charged with 9.20 × 10-3 mol of 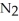
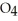 .At equilibrium,5.98 × 10-3 mol of
.At equilibrium,5.98 × 10-3 mol of 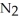
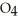 remains.
remains. 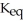 for this reaction is ________.
for this reaction is ________.
Definitions:
Related Questions
Q1: Which of the following expressions is the
Q11: The _ for Ge shows it to
Q18: _ generally differ from compounds in that
Q36: A particular first-order reaction has a rate
Q58: Which solution will be the most basic?<br>A)0.10
Q63: Ammonia is a _.<br>A)weak acid<br>B)strong base<br>C)weak base<br>D)strong
Q67: The concentration of Na in seawater is
Q69: What is the molarity of phosphoric acid
Q79: If the reaction quotient Q for a
Q114: The mole fraction of He in a