At 900.0 K,the equilibrium constant (Kp) for the following reaction is 0.345. 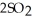 +
+ 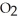 (g) →
(g) → 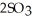 (g) At equilibrium,the partial pressure of
(g) At equilibrium,the partial pressure of 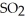 is 36.9 atm and that of
is 36.9 atm and that of 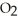 is 16.8 atm.The partial pressure of
is 16.8 atm.The partial pressure of 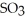 is ________ atm.
is ________ atm.
Definitions:
Economic Efficiency
A situation where resources are allocated in a way that maximizes the production of goods and services at the lowest cost.
Market
A place or arrangement through which goods and services are exchanged between buyers and sellers.
Public Good
A commodity or service that is provided without profit to all members of a society, either by the government or by a private individual or organization.
Nonrival-in-consumption
A characteristic of goods where one person's consumption does not diminish the ability of others to use the same good.
Q3: The vapor pressure of pure water at
Q4: The value of K<sub>eq</sub> for the equilibrium
Q19: Which group of transition metals have the
Q50: Which one of the following vitamins is
Q70: The value of ΔG° at 25 °C
Q89: Consider the following reaction: A → B<br>The
Q109: Of the reactions involved in the photodecomposition
Q121: The primary detrimental effect of the presence
Q153: The solubility of Ar in water at
Q159: Water (H<sub>2</sub>O)and the alcohol methanol (CH<sub>3</sub>OH)are infinitely