Consider the following reaction at equilibrium: 2C 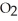 (g)
(g)  2CO (g) +
2CO (g) + 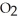 (g) ΔH° = -514 kJ
(g) ΔH° = -514 kJ
Le Châtelier's principle predicts that removing 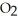 (g) to the reaction container will ________.
(g) to the reaction container will ________.
Definitions:
Producer Surplus
The difference between what producers are willing to accept for a good versus what they actually receive, measured above the supply curve.
Market Price
The existing price point at which an asset or service is traded in a designated market area.
Market
A system through which buyers and sellers interact to trade goods, services, or resources.
Law Of Supply
The principle that, all else equal, an increase in the price of a good will result in an increase in the quantity supplied.
Q3: The K<sub>eq</sub> for the equilibrium below is
Q13: The reaction CH<sub>3</sub>-N≡C → CH<sub>3</sub>-C≡N<br>Is a first-order
Q53: A reaction was found to be second
Q54: What happens to a polymer as it
Q66: The initial discovery of a liquid crystal
Q67: The concentration of Na in seawater is
Q71: Given the following reaction at equilibrium at
Q88: The rate of a chemical reaction should
Q100: A 25.0 mL sample of 0.723 M
Q128: The Earth's ozone layer is located in