What is the pOH of a 0.20 M aqueous solution of N 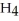 Br at 25.0 °C?
Br at 25.0 °C? 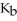 for N
for N 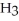 is 1.8 ×
is 1.8 × 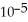 .
.
Definitions:
Carbon Atoms
The basic building blocks of the vast majority of organic compounds, capable of forming strong covalent bonds due to their tetravalency.
Hydrogen Atoms
The simplest type of atoms consisting of one proton and one electron, fundamental in chemistry and the building blocks of molecules.
Alkane Name
The systematic nomenclature assigned to alkanes based on the number of carbon atoms in their longest chain and specific rules.
Triacontane
A saturated hydrocarbon with a chemical formula of C30H62, commonly found in waxes.
Q9: The process of solute particles being surrounded
Q10: The value of ΔH° for the formation
Q24: Which of the following statements is false?<br>A)The
Q42: The value of ΔG° at 25 °C
Q63: The normal boiling point of water is
Q70: A water sample tested positive for lead
Q73: As the temperature of a reaction is
Q74: Which one of the following processes produces
Q75: Consider the reaction: FeO (s)+ Fe (s)+
Q95: The stratosphere contains approximately _ of the