Calculate the pH of a solution prepared by dissolving 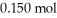 of benzoic acid and
of benzoic acid and 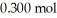 of sodium benzoate in water sufficient to yield
of sodium benzoate in water sufficient to yield 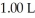 of solution.The Ka of benzoic acid is
of solution.The Ka of benzoic acid is 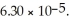
Definitions:
Rate Difference
The disparity in interest rates, often used in the context of comparing rates between two different financial products or within different markets.
Spread
The difference between the bid and the ask price of a security or the difference between the interest rate paid for deposits and received for loans.
Institutional Investors
Organizations such as banks, insurance companies, and pension funds that make substantial investments on the capital markets.
Major Exchanges
The leading stock exchanges worldwide, such as the New York Stock Exchange and the Nasdaq, where securities are bought and sold.
Q2: Which of the following expressions is the
Q5: What is the percentage of freshwater on
Q25: What is the molality of a 36.1%
Q30: ΔS is positive for the reaction _.<br>A)2NO
Q34: Calculate the pH of a solution at
Q59: What is the pOH of a sodium
Q61: As one gains altitude in the atmosphere,based
Q91: For the endothermic reaction CaCO<sub>3</sub> (s) <img
Q91: The value of ΔS° for the formation
Q116: Which of the following could be added