Calculate the pH of a solution prepared by dissolving 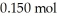 of acetic acid and
of acetic acid and 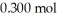 of sodium acetate in water sufficient to yield
of sodium acetate in water sufficient to yield 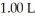 of solution.The Ka of acetic acid is
of solution.The Ka of acetic acid is 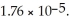
Definitions:
Total Sales Revenue
The sum of all income generated by the sales of goods or services by a company before any costs or expenses are deducted.
Expectancy Theory
A motivational theory that suggests an individual's behavior is determined by their expected rewards or outcomes.
Performance Bonus
A supplementary compensation awarded to an employee for achieving or surpassing specific goals.
Executive Coaching
A personal developmental process where an experienced coach supports an executive or leader to achieve specific professional goals or improve leadership skills.
Q2: The elementary reaction 2NO<sub>2</sub> (g)→ 2NO (g)+
Q28: In the coal-gasification process,carbon monoxide reacts with
Q49: The concentration of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The concentration
Q53: A reaction was found to be second
Q53: Which reaction produces an increase in the
Q60: Using the data in the table,which of
Q78: Which of the following has the largest
Q99: Calculate the pH of a solution prepared
Q103: What is meant by the salinity of
Q137: A- is a weak base.Which equilibrium corresponds