What is the pH of a buffer solution that is 0.172 M in hypochlorous acid (HClO) and 0.131 M in sodium hypochlorite? The 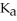 of hypochlorous acid is 3.8 ×
of hypochlorous acid is 3.8 × 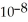 .
.
Definitions:
Salary
A regular payment, typically monthly or biweekly, given by an employer to an employee, especially a professional or a white-collar worker.
Plan
A detailed proposal for doing or achieving something, often involving the consideration of objectives, strategies, and tactics.
Sleeper Effect
A delayed increase in the impact of a message, where an initially disregarded or trivialized message becomes influential over time.
Persuasion
The process of influencing someone's beliefs, attitudes, intentions, motivations, or behaviors.
Q6: The reaction that forms most of the
Q38: The equilibrium constant for reaction 1 is
Q39: Units of the rate constant of a
Q40: At constant _ and _ the Gibbs
Q52: The average rate of disappearance of A
Q52: What is the molal concentration of potassium
Q58: The half-life of <sup>131</sup>I is 0.220 years.How
Q117: Ozone is more efficient at killing bacteria
Q122: The conjugate base of HPO<sub>4</sub><sup>2-</sup> is _.<br>A)PO<sub>4</sub><sup>3-</sup><br>B)H<sub>2</sub>PO<sub>4</sub><br>C)H<sub>3</sub>PO<sub>4</sub><br>D)H<sub>2</sub>PO<sub>4</sub><sup>-</sup><br>E)none
Q170: Which of these nuclides is most likely