Calculate the pH of a solution prepared by dissolving 0.250 mol of benzoic acid ( 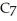
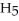
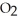 H) and
H) and 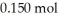 of sodium benzoate (Na
of sodium benzoate (Na 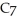
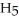
 ) in water sufficient to yield 1.00 L of solution.The
) in water sufficient to yield 1.00 L of solution.The 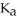 of benzoic acid is
of benzoic acid is 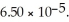
Definitions:
Stimuli
External agents or occurrences that provoke a reaction or response from a biological organism.
Asthmatics
are individuals who suffer from asthma, a chronic respiratory condition characterized by episodes of wheezing, coughing, and difficulty breathing.
Cannon-Bard Theory
A theory of emotion that argues that physiological arousal and emotional experience occur simultaneously, yet independently.
Physiological Symptoms
Observable body responses to various stimuli, indicating a reaction, such as increased heart rate, sweating, or a rash, in relation to emotions, disease processes, or environmental factors.
Q35: The equilibrium constant for the gas phase
Q36: Nitrogen oxides catalytically destroy ozone.
Q49: If the rate law for the reaction
Q60: The concentration of reactants or products at
Q77: Which one of the following pairs cannot
Q81: An aqueous solution at 25.0°C contains [
Q95: A sealed 1.0 L flask is charged
Q105: Which one of the following is used
Q106: What is the order of the reaction
Q107: What is the pH of a sodium