A solution is prepared by dissolving 0.23 mol of hypochlorous acid and 0.27 mol of sodium hypochlorite in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly.The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution.The 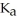 of hypochlorous acid is 1.36 × 10-3.
of hypochlorous acid is 1.36 × 10-3.
Definitions:
Q6: Using the data in the table,which of
Q15: What is the molar solubility of silver
Q17: A 50.0 mL sample of an aqueous
Q39: A voltaic cell is constructed with two
Q46: The molar concentration of hydronium ion in
Q51: The mass of a proton is 1.00728
Q104: What is the total pressure (torr)inside a
Q112: What is the pOH of an aqueous
Q115: The pH of a 0.25 M aqueous
Q140: Which one of the following is a