Calculate the pH of a solution prepared by dissolving 0.270 mol of weak acid HA and 0.260 mol of its conjugate base in water sufficient to yield 1.00 L of solution.The 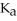 of HA is
of HA is 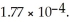
Definitions:
Net Book Value
The value of an asset as recorded on the balance sheet, subtracting accumulated depreciation or amortization from its original cost.
Depreciation
The systematic allocation of the cost of a tangible asset over its useful life, reflecting the consumption or wear and tear of the asset.
Carrying Value
Also known as book value, it is the value at which an asset is recognized in the balance sheet after accounting for depreciation, amortization, and impairment charges.
Consolidated Financial Statements
Combined financial statements of a parent company and its subsidiaries, presenting the financial position and results of operations of these separate entities as a single economic entity.
Q11: Of the following,_ will lower the activation
Q18: In the electrochemical cell using the redox
Q33: The concept that radiowave propagation was affected
Q36: Nitrogen oxides catalytically destroy ozone.
Q36: A 0.5 M solution of _ has
Q48: What is the pH of a 0.40
Q64: Transuranium elements have atomic numbers greater than
Q70: Sulfur released into the troposphere arises from
Q82: Which reaction will shift to the right
Q94: If ΔG° for a reaction is less