A 25.0 mL sample of 0.723 M HCl 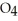 is titrated with a
is titrated with a 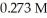 KOH solution.The
KOH solution.The 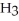
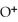 concentration after the addition of
concentration after the addition of 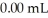 of KOH is ________ M.
of KOH is ________ M.
Definitions:
Nissan
A multinational automobile manufacturer headquartered in Japan, known for its wide range of cars, trucks, and electric vehicles.
Cross-Border Mergers
Transactions where companies from different countries combine their operations or assets, often to expand market reach or leverage strategic advantages.
Direct Foreign Investment
A financial commitment made by a company or individual in one country to business interests in another country, typically via acquiring business assets or establishing business operations.
Differing Languages
The existence or use of distinct linguistic systems or dialects within communication that can lead to diversity in expression and misunderstanding.
Q2: The conjugate base of HSO<sub>4</sub><sup>-</sup> is _.<br>A)H<sub>2</sub>SO<sub>4</sub><br>B)HSO<sub>4</sub><sup>+</sup><br>C)H<sup>+</sup><br>D)SO<sub>4</sub><sup>2-</sup><br>E)HSO<sub>3</sub><sup>+</sup>
Q8: How many grams of solution are present
Q10: Hydrogen and iodine gas react to produce
Q32: What is the pOH of an aqueous
Q39: Pure solids and pure _ are excluded
Q48: Which of the following has the largest
Q82: What is the oxidation number of manganese
Q84: The combustion of hydrogen in the presence
Q98: Which of the following reactions would have
Q103: The rate limiting step in a reaction