Given the thermodynamic data in the table below,calculate the equilibrium constant (at 298 K) for the reaction: 2 SO2 (g) + O2 (g)  2 SO3 (g)
2 SO3 (g) 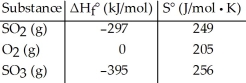
Definitions:
Intravascular
Occurring within the blood vessels; refers to processes or treatments administered directly into the circulatory system.
Diuretic
A type of medication that helps remove excess fluid from the body by increasing urine production, commonly used in treating high blood pressure and edema.
Electrolyte Imbalance
A condition where the levels of electrolytes in the body are either too high or too low, impacting various bodily functions.
Sodium Imbalance
An irregular level of sodium in the blood, which can lead to various health problems such as dehydration or fluid retention.
Q24: Which one of the following is not
Q26: Which group 3A element is a metalloid?<br>A)B<br>B)Al<br>C)Ga<br>D)In<br>E)Tl
Q86: The relationship between the change in Gibbs
Q96: A 25.0 mL sample of 0.150 M
Q100: The standard cell potential (E°<sub>cell</sub>)for the voltaic
Q126: Which of the following compounds would produce
Q132: What is the conjugate acid of HCO<sub>3</sub><sup>-</sup>?<br>A)CO<sub>2</sub><sup>2-</sup><br>B)H<sub>2</sub>CO<sub>3</sub><br>C)HCO<sub>2</sub><sup>2-</sup><br>D)CO<sub>3</sub><sup>2-</sup><br>E)none
Q137: The Haber process is used to make
Q142: Which equation correctly represents the reaction between
Q150: The basis for the carbon-14 dating method