For the reaction C(s) + 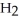 O(g) → CO(g) +
O(g) → CO(g) + 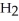 (g)
(g)
ΔH° = 133.3 kJ/mol and ΔS° = 121.6 J/K ∙ mol at 298 K.At temperatures greater than ________ °C this reaction is spontaneous under standard conditions.
Definitions:
Resale Price Maintenance
A practice where a manufacturer or other supplier dictates the minimum price at which retailers are allowed to sell its product.
Volume-Based Product Costing
A costing methodology that assigns costs to products based on the volume of units produced, utilizing overhead rates calculated from total production volume.
Marginal Revenue
The additional income generated from selling one more unit of a good or service.
Marginal Cost Paradigm
The economic principle that examines the additional costs incurred from producing one more unit of a good or service.
Q7: The extent of ionization of a weak
Q18: In the electrochemical cell using the redox
Q25: What is the major commercial source of
Q27: What is the oxidation number of oxygen
Q29: What is the coefficient of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg"
Q41: Which of the following could be added
Q46: The more negative ΔG° is for a
Q101: The concentration of which greenhouse gas has
Q104: How many kilowatt-hours of electricity are used
Q194: SiO<sub>4</sub><sup>4-</sup> is the _ ion.<br>A)orthosilicate ion<br>B)silicide ion<br>C)thiosilicate