The equilibrium constant for the following reaction is 3.0 × 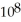 at 25 °C.
at 25 °C. 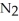 (g) + 3
(g) + 3 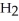 (g)
(g)  2N
2N 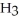 (g) The value of ΔG° for this reaction is ________ kJ/mol.
(g) The value of ΔG° for this reaction is ________ kJ/mol.
Definitions:
Livestock
Domesticated animals raised in an agricultural setting to produce labor and commodities such as meat, eggs, and milk.
Environmental Effect
The impact of human activities or natural processes on the ecological balance or health of the biosphere.
Subsistence Farming
A type of agriculture where farmers grow food crops to meet the needs of themselves and their families, with little to no surplus.
Large-Scale Farming
Agricultural operations that involve vast areas of land and significant levels of production, often utilizing industrial methods.
Q48: The prefix thio- denotes _.<br>A)replacement of an
Q57: What is the coefficient of NO<sub>2</sub> when
Q63: The addition of KOH and _ to
Q102: In metallic hydrides,the oxidation number of hydrogen
Q110: An aqueous solution of NaF is prepared
Q116: What is the equilibrium constant for a
Q130: What is the atomic number of a
Q142: Which equation correctly represents the reaction between
Q166: The correct name for NaBH<sub>4</sub> is _.<br>A)sodium
Q194: SiO<sub>4</sub><sup>4-</sup> is the _ ion.<br>A)orthosilicate ion<br>B)silicide ion<br>C)thiosilicate