The standard cell potential (E°cell) of the reaction below is +1.34 V.The value of 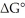 for the reaction is ________ kJ/mol. 3 Cu (s) + 2 MnO4- (aq) + 8H+ (aq) → 3 Cu2+ (aq) + 2 MnO2 (s) + 4 H2O (l)
for the reaction is ________ kJ/mol. 3 Cu (s) + 2 MnO4- (aq) + 8H+ (aq) → 3 Cu2+ (aq) + 2 MnO2 (s) + 4 H2O (l)
Definitions:
Free Association
A psychoanalytic technique in which clients are encouraged to express freely any thoughts, feelings, or images that come to mind, without censorship or filtering.
Aversive Conditioning
A behavioral conditioning technique where an unpleasant stimulus is associated with undesirable behavior in order to reduce or eliminate that behavior.
Flooding
Flooding is a psychological treatment method for phobias and anxiety disorders where an individual is exposed to an overwhelming amount of the feared stimulus in order to extinguish the fear response.
Hypochondriasis
A condition characterized by an excessive preoccupation with the fear of having a serious disease.
Q27: What is meant by the term "composite"?
Q84: Only the most active metals react with
Q85: What happens to the mass number and
Q98: The pOH of a 0.25 M aqueous
Q99: Define a coulomb.
Q106: Which of the following cannot form both
Q112: The less _ the value of E°<sub>red</sub>,the
Q119: What is the concentration (in M)of hydronium
Q126: The decay of a radionuclide with a
Q154: Dissolving 2.0 mol of _ in 1.0