In the galvanic cell using the redox reaction below,the reduction half-reaction is ________. Zn (s) + 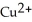 (aq) →
(aq) → 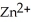 (aq) + Cu (s)
(aq) + Cu (s)
Definitions:
Government
The organization, or system by which a community or nation is governed, responsible for creating and enforcing laws.
Consumer Surplus
The offset between what customers are willing and able to pay for a service or product and what they actually expend.
Consumer Surplus
The distinction between the ideal spending amount of consumers on a good or service and their actual expenditure.
Government
The organization that is the governing authority of a political unit, responsible for creating and enforcing laws and managing public services.
Q17: _ discovered radioactivity.
Q20: A 25.0 mL sample of a solution
Q35: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="The for
Q37: Which one of the following is always
Q75: Consider the reaction: FeO (s)+ Fe (s)+
Q76: The oxidation state of fluorine in its
Q78: Photoionization processes (e.g.,N<sub>2</sub> + hν → N<sub>2</sub><sup>+</sup>
Q91: The value of ΔS° for the formation
Q97: The brown color of photochemical smog over
Q149: In the nuclear transmutation represented by <br><img