The standard cell potential (E°cell) of the reaction below is -0.55 V.The value of 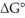 for the reaction is ________ J/mol. I2 (s) + 2Br- (aq) → 2I- (aq) + Br2 (l)
for the reaction is ________ J/mol. I2 (s) + 2Br- (aq) → 2I- (aq) + Br2 (l)
Definitions:
Generalist Countries
Refers to nations with policies or economies that are not specialized but rather encompass a broad range of industries and activities.
Multinational Corporations
Large companies that operate in multiple countries, managing production or delivering services in more than one country.
Job Mobility
the ability of workers to move between different jobs, roles, or industries, often as a way to advance their careers or improve their work situation.
Nationally Focused
Concentrating efforts, policies, or interests on issues within a single country rather than on international perspectives or concerns.
Q23: What are the three steps in the
Q30: Calculate the pH of a buffer solution
Q39: Which of the following would produce the
Q47: Consider an electrochemical cell based on the
Q53: Which reaction produces an increase in the
Q80: Which substance is the oxidizing agent in
Q105: A decrease in the entropy of the
Q123: Complexes containing metals with which one of
Q139: Which of the following ions can form
Q162: The missing product in this reaction would