The standard cell potential (E°cell) of the reaction below is -0.34 V.The value of 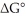 for the reaction is ________ kJ/mol. Cu (s) + 2H+ (aq) → Cu2+ (aq) + H2 (g)
for the reaction is ________ kJ/mol. Cu (s) + 2H+ (aq) → Cu2+ (aq) + H2 (g)
Definitions:
Group Processors
Individuals or mechanisms that manage, direct, or facilitate the work within a group towards achieving collective goals.
Interlocking Networks
A pattern of overlapping relationships and connections among people or organizations, which can facilitate information flow and collaboration.
Monopolistically Competitive
A type of market structure characterized by many sellers offering differentiated products, leading to competition based on product features, price, and quality.
Profit
The financial gain obtained when the amount of revenue gained from a business activity exceeds the expenses, costs, and taxes needed to sustain the activity.
Q3: The amount of fissionable material necessary to
Q56: The carbon-14 dating method can be used
Q68: The dividing line between the troposphere and
Q69: How many milliliters of 0.0839 M NaOH
Q82: The value of ΔS° for the oxidation
Q99: Calculate the molarity of hydroxide ion in
Q113: Which mineral contains titanium?<br>A)pyrolusite<br>B)chalcopyrite<br>C)galena<br>D)rutile<br>E)sphalerite
Q141: Which equation correctly represents the reaction between
Q176: The number of electrons in the valence
Q183: Boric acid condenses to form tetraboric acid