The standard cell potential (E°cell) of the reaction below is +1.34 V.The value of 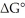 for the reaction is ________ kJ/mol. 3 Cu (s) + 2 MnO4- (aq) + 8H+ (aq) → 3 Cu2+ (aq) + 2 MnO2 (s) + 4 H2O (l)
for the reaction is ________ kJ/mol. 3 Cu (s) + 2 MnO4- (aq) + 8H+ (aq) → 3 Cu2+ (aq) + 2 MnO2 (s) + 4 H2O (l)
Definitions:
Controllable Fixed Costs
Expenses that can be managed or influenced by decisions made by a company's management, such as advertising expenditures.
Operating Assets
Assets used by a business in its day-to-day operations to generate revenue.
Controllable Margin
A financial metric that represents the amount of profit or contribution margin that management can directly influence through its decisions.
Operating Assets
Assets that a business uses for its day-to-day operational activities to generate revenue, excluding investment and non-operational assets.
Q2: Complexes containing metals with d<sup>10</sup> electron configurations
Q13: The value of ΔS° for the decomposition
Q33: The beta decay of cesium-137 has a
Q50: Changes in the coordination sphere of a
Q81: The coordination number of platinum in complexes
Q96: The most common oxidation state of silicon
Q97: What is the mass defect (in amu)of
Q119: The correct name of H<sub>2</sub>CO<sub>3</sub> is _.<br>A)hydrogen
Q153: Addition of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="Addition of
Q167: What is emitted in the nuclear transmutation,