In the galvanic cell using the redox reaction below,the reduction half-reaction is ________. Zn (s) + 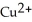 (aq) →
(aq) → 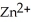 (aq) + Cu (s)
(aq) + Cu (s)
Definitions:
Janice Radway
A scholar known for her contributions to the fields of cultural studies and American literature, especially for her work on reading communities.
Romance Novels
A literary genre focused on the theme of romantic love, typically with a plot that centers on a romantic relationship.
Female Power
signifies the influence and authority that women possess and exercise in various spheres of life, including social, political, and economic realms.
Promiscuity
A term often used to describe a pattern of engaging in multiple sexual relationships or encounters in a short period of time, without commitment.
Q22: The coordination number and oxidation number of
Q23: Which one of the following is produced
Q66: K<sub>a</sub> for arsenic acic,HAsO<sub>4</sub><sup>2-</sup>,is 7.5 × 10<sup>-12</sup>.What
Q75: What are beta particles?
Q76: Which one of the following is not
Q76: The conjugate base of NH<sub>3</sub> is _.<br>A)NH<sub>2</sub><sup>-</sup><br>B)NH<sub>4</sub><sup>+</sup><br>C)NH<sub>2</sub>OH<br>D)H<sub>3</sub>O<sup>+</sup><br>E)OH<sup>-</sup>
Q77: Which of the following statements is true?<br>A)Processes
Q105: A decrease in the entropy of the
Q115: Clean rainwater is _ mainly due to
Q160: What percentage of electricity generated in the