Based on the following information,calculate the mass of one atom of 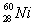 . mass of an electron = 9.110 x 10-28 g
. mass of an electron = 9.110 x 10-28 g
Mass of a proton = 1.673 x 10-24 g
Mass of a neutron = 1.675 x 10-24 g
Definitions:
Hard Cheese
A type of cheese that has a firm texture and is often aged longer, resulting in a sharper and more pronounced flavor.
Methylphenidate
A central nervous system stimulant medication commonly used to treat Attention Deficit Hyperactivity Disorder (ADHD) and certain sleep disorders.
Nervousness
The feeling of being anxious, tense, or worried, often in anticipation of an event or in response to a stressful situation.
Daytime Sleepiness
A condition characterized by feeling unusually sleepy during the day, which can be a symptom of sleep disorders or other health issues.
Q7: How many moles are present in 1.64g
Q23: How many molecules are present in 0.340g
Q28: Define in simple terms the concept of
Q34: The alkali metals are in group<br>A)1A<br>B)3A<br>C)5A<br>D)7A
Q40: How many units of MgSO<sub>4</sub> are present
Q50: What is meant by the terms atom
Q74: Which is a metalloid?<br>A)Li<br>B)Be<br>C)B<br>D)C
Q79: A compound has the formula Sr<sub>3</sub>Y<sub>2</sub>.Which of
Q98: The specific heat of copper is 0.386
Q105: How many different elements are present in