Rubidium occurs naturally as two isotopes: 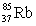 with a mass of 84.9117 amu and
with a mass of 84.9117 amu and 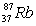 with a mass of 86.9092 amu.Given that the atomic mass of rubidium is 85.47 amu,what are the natural abundances of the two isotopes?
with a mass of 86.9092 amu.Given that the atomic mass of rubidium is 85.47 amu,what are the natural abundances of the two isotopes?
Definitions:
Relevant Costs
Costs that are directly related to a specific management decision and that will change as a result of that decision.
Sunk Costs
Costs that have already been incurred and cannot be recovered, which should not affect future business decisions.
Alternatives
Different options or strategies available for achieving a goal or solving a problem, often considered during decision-making processes.
Total Fixed Cost
The sum of all costs that remain constant regardless of the level of production or business activity.
Q1: A sample of water releases 4.50 x
Q25: The prefix "tetra" means four.
Q31: All of the following are used to
Q33: H<sub>2</sub>SO<sub>3</sub> is<br>A)hydrosulfuric acid.<br>B)sulfuric acid.<br>C)hydrosulfurous acid.<br>D)sulfurous acid.
Q40: The number of waves that pass a
Q48: Express the number 2.64 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4036/.jpg" alt="Express
Q58: The mass number of an atom is
Q67: Name these compounds formed between nitrogen and
Q75: Which is a characteristic of all nonmetals?<br>A)Always
Q98: When expressed in proper scientific notation the