Based on the following information,calculate the mass of one atom of 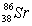 . mass of an electron = 9.110 x 10-28 g
. mass of an electron = 9.110 x 10-28 g
Mass of a proton = 1.673 x 10-24 g
Mass of a neutron = 1.675 x 10-24 g
Definitions:
Normal Yield Curve
A graphical representation that shows interest rates on bonds of different maturities tend to increase the longer the term to maturity, under normal market conditions.
Expected Inflation
The anticipated rate at which the general level of prices for goods and services will rise over a period.
Expectations Theory
A theory that explains the term structure of interest rates based on the anticipation that the rates will move toward the average rate over the long term.
Yield Curve
A graph showing the relationship between bond yields and their maturities, often used to predict changes in economic output and interest rates.
Q12: In the reaction: CuSO<sub>4</sub> + BaBr<sub>2</sub>
Q12: The specific heat of zinc is 0.388
Q13: Divide 1436 by 203.The quotient expressed to
Q33: How many moles of CO<sub>2</sub> are
Q66: Tandem Company borrowed $32,000 of cash from
Q68: In nature most matter exists as pure
Q68: The following reaction: Ca + 2H<sub>2</sub>O
Q72: Which of the following reactants would you
Q90: Which represents an element?<br>A)CO<br>B)NI<br>C)N<sub>2</sub><br>D)OS
Q126: Packard Company's net cash flow from financing