Rubidium occurs naturally as two isotopes: 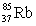 with a mass of 84.9117 amu and
with a mass of 84.9117 amu and 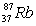 with a mass of 86.9092 amu.Given that the atomic mass of rubidium is 85.47 amu,what are the natural abundances of the two isotopes?
with a mass of 86.9092 amu.Given that the atomic mass of rubidium is 85.47 amu,what are the natural abundances of the two isotopes?
Definitions:
RRSP fund
A Registered Retirement Savings Plan (RRSP) fund is a retirement savings plan sponsored by the Canadian government, allowing citizens to save for retirement on a tax-sheltered basis.
Nominal rate
An interest rate or return quoted on an investment or loan, not adjusted for inflation or other factors.
Effective rate
The total compounded interest rate, expressed annually, that represents the actual financial cost of a loan or investment.
Compounded quarterly
Interest is recalculated every three months based on both the initial principal and previously earned interest, leading to exponential growth of an investment or debt.
Q2: Given the unbalanced equation: CH<sub>4</sub> +
Q8: Given the unbalanced equation: CO<sub>2</sub>
Q12: In isotopic notation atomic number is represented
Q32: A cube measures 13.00cm on edge.What is
Q41: How many moles of CO<sub>2</sub> are
Q64: The charge of an anion is<br>A)positive.<br>B)negative.<br>C)neutral.
Q64: In a reaction to produce iron the
Q65: All ions have a charge of zero.
Q96: On the periodic table,the "representative elements" are
Q104: A combustion reaction will always involve the