Calculate the number of molecules present in 122 g of the compound shown below. 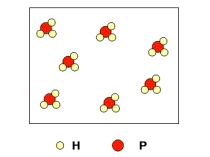
Definitions:
Boiling Points
The temperature at which a substance transitions from the liquid phase to the gas phase at atmospheric pressure.
Isomers
Substances that share the same molecular formula, yet differ in the arrangement of their atoms, resulting in distinct physical and chemical characteristics.
Acetylene
A colorless hydrocarbon with the formula C2H2, characterized by a triple carbon-carbon bond, used as a fuel and a chemical building block.
Molecular Dipole Moment
A measure of the charge distribution within a molecule, significant in predicting the molecule’s behavior in electric fields and solvent interactions.
Q5: Assume equal masses of the following substances,all
Q18: What is the maximum number of electrons
Q23: For each of the following compounds,choose its
Q23: What is the formula of magnesium sulfide?<br>A)Mg<sub>2</sub>S<br>B)MgS<sub>2</sub><br>C)MgS<br>D)Mg<sub>3</sub>S
Q28: As the difference in electronegativity between two
Q37: Which is an alkaline earth metal?<br>A)Hydrogen<br>B)Magnesium<br>C)Manganese<br>D)Iodine
Q52: Complete and balance the equation for
Q64: A 120.mL sample of a gas is
Q80: Which is phosphorous pentachloride?<br>A)PCl<sub>5</sub><br>B)P<sub>5</sub>Cl<br>C)P<sub>2</sub>Cl<sub>5</sub><br>D)P<sub>5</sub>Cl<sub>2</sub>
Q111: How many molecules are present in 4.00