Based on the following chemical equation 2C6H6 + 15O2 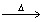 12CO2 + 6H2O
12CO2 + 6H2O
Calculate the number of moles of CO2 that will be produced when 8.90 moles of H2O are produced as well?
Definitions:
Third-party Beneficiary
An individual or legal entity that gains a benefit from a contract despite not being one of the primary parties involved.
Creditor Beneficiary
A third party that benefits from a contract wherein the promisor agrees to pay the promisee's debt to the beneficiary.
Donee Beneficiary
A third party who stands to benefit from a contract, especially when the contract is intended as a gift to that third party.
Assignor
An individual or entity that transfers rights or interests in a contract to another, known as the assignee.
Q11: Given the unbalanced equation: H<sub>2</sub>O
Q13: What volume of ammonia is produced
Q19: As the volatility of a liquid increases,its
Q20: All of the following contain the same
Q25: In the expression,3CaBr<sub>2</sub>,<br>A)2 and 3 are subscripts<br>B)2
Q53: Which is a basic anhydride?<br>A)Sodium oxide<br>B)Nitrogen dioxide<br>C)Dinitrogen
Q54: The shape of a carbon dioxide molecule
Q72: How many moles of silver ions are
Q83: As Kelvin temperature increases,the average kinetic energy
Q103: Melting and boiling are examples of physical