How many moles of benzene (C6H6) should be completely combusted in order to produce 0.4000 moles of carbon dioxide? 2C6H6 + 15O2 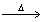 12CO2 + 6 H2O
12CO2 + 6 H2O
Definitions:
Transactions
The exchange of goods, services, or funds between two or more parties, forming the basis of accounting records.
Debit Credit
The two aspects of every financial transaction, where debit increases assets or expenses and credit increases liabilities, equity, or income.
Par Value
The nominal value of a bond, share of stock, or coupon as stated by the issuer, often used as a legal face value.
Common Stock
Holding shares in a company grants investors a portion of ownership, allowing them to vote on corporate matters and receive a portion of the profits via dividends.
Q2: Three different substances are placed in three
Q35: Which is a cation?<br>A)A vanadium (II)ion<br>B)A fluoride
Q39: What is the molar mass of a
Q46: Sulfur dioxide reacts with oxygen to produce
Q56: Halogens generally form ions with a charge
Q61: What is the percent by mass of
Q68: As the average kinetic energy of the
Q81: How many moles of Al(OH)<sub>3</sub> are
Q85: What is the mass number of an
Q100: Which is a basic anhydride?<br>A)Carbon dioxide<br>B)Carbon monoxide<br>C)Sulfur