Consider the single-displacement reaction in which zinc metal displaces hydrogen from hydrochloric acid.
A.Write a complete and balanced chemical equation for this reaction.
B.If 3.4
 1023 zinc atoms react with 4.5
1023 zinc atoms react with 4.5
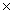 1023 molecules of hydrochloric acid,calculate the mass of zinc chloride produced in this reaction.
1023 molecules of hydrochloric acid,calculate the mass of zinc chloride produced in this reaction.
C.Calculate the number of moles of the excess reactant that remain once the reaction stops.
Definitions:
Financial Condition
Refers to the current state of a company's financial health and stability, often assessed through analyzing balance sheets, income statements, and cash flow statements.
Par Value
The nominal or face value of a security or stock as stated by the issuing company, which might not reflect its market value.
Book Value
The value of an asset according to its balance sheet account balance, which is the cost of the asset minus any depreciation, amortization, or impairment costs made against the asset.
Q18: An atom of gallium has a mass
Q22: Which isotope contains the same number of
Q22: What volume of carbon dioxide gas
Q41: How many moles of CO<sub>2</sub> are
Q49: One liter of 2.0 M KCl solution
Q56: How many molecules are present in 0.300
Q80: As the pressure of a sample of
Q84: Which compound's name ends in -ate?<br>A)H<sub>2</sub>SO<sub>3</sub><br>B)CaS<br>C)MgSO<sub>4</sub><br>D)HClO
Q99: The shape of an ammonia molecule is<br>A)bent.<br>B)trigonal
Q100: The volume of a gas must always