Based on the following chemical equation 2C6H6 + 15O2 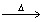 12CO2 + 6H2O
12CO2 + 6H2O
Calculate the number of moles of CO2 that will be produced when 8.90 moles of H2O are produced as well?
Definitions:
Callable Bonds
Bonds that can be redeemed by the issuer before their maturity date at a predetermined call price.
Stated Dollar Amount
A specific monetary value declared or agreed upon, which is often used in financial contexts for contracts or transactions.
Par Value
The face value of a bond or the stock value stated in the corporate charter, often set at a minimal amount.
Market Value
The current price at which an asset or service can be bought or sold in the market, not necessarily equal to the book value.
Q15: Which has the lowest vapor pressure?<br>A)25 mL
Q22: What volume of carbon dioxide gas
Q28: Natural gas is primarily composed of<br>A)butane.<br>B)propane.<br>C)octane.<br>D)methane.
Q30: A sample of gas has a volume
Q32: Calculate the number of moles of excess
Q35: How many chloride ions are present in
Q41: All the following contain the same number
Q51: A small,0.500 mL,bubble forms at the bottom
Q53: CuCl<sub>2</sub> is<br>A)copper (I)chloride.<br>B)copper (II)chloride.<br>C)copper chloride (I).<br>D)copper chloride
Q113: When the equation Cr<sub>2</sub>S<sub>3</sub> + HCl