How many moles of benzene (C6H6) should be completely combusted in order to produce 0.4000 moles of carbon dioxide? 2C6H6 + 15O2 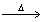 12CO2 + 6 H2O
12CO2 + 6 H2O
Definitions:
New Middle Class
A socio-economic group that emerged in industrial societies, characterized by higher education levels, professional occupations, and relatively high income.
Physicians
Medical professionals who diagnose, treat, and help prevent diseases and injuries, typically holding a degree in medicine.
Free Black Americans
African Americans who were not enslaved and lived freely in the United States, especially significant before the Civil War in the context of a society divided by slavery.
Q13: Which is plumbous chloride?<br>A)PbCl<br>B)PbCl<sub>2</sub><br>C)PbCl<sub>3</sub><br>D)PbCl<sub>4</sub>
Q24: In isotopic notation mass number is represented
Q32: What is the molecular formula of a
Q34: Which contains both ionic and covalent bonds?<br>A)(KCl)<br>B)(NH<sub>3</sub>
Q40: A sample of gas is in a
Q45: How many moles are present in 0.140g
Q51: Which compound contains a triple bond in
Q85: The following reaction: 2Al + 3O<sub>2</sub>
Q93: The atomic radius generally decreases as we
Q99: What is the formula for the compound