Calculate how much ethanol,C2H5OH,will be produced from the reaction of 590.g of sugar (C6H12O6) if the reaction has a 88.5% yield. C6H12O6 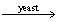 2C2H5OH + 2CO2
2C2H5OH + 2CO2
Definitions:
Price-Elastic
A characteristic of a good or service that indicates the demand for it changes significantly when its price changes.
Demand Pay
Compensation determined by the desirability or demand for specific job skills in the labor market.
More Elastic
Refers to a greater sensitivity or responsiveness of the quantity demanded or supplied to changes in price, indicating a more noticeable shift in consumer or producer behavior in response to price fluctuations.
Senior Citizens
Individuals typically aged 65 and over who may qualify for certain benefits and considerations due to their age.
Q4: A Ca<sup>+2</sup> ion has an electron configuration
Q7: At STP,which sample contains twice the number
Q24: Hydrogen bonds form between molecules of<br>A)H<sub>2</sub>O<br>B)H<sub>2</sub>S<br>C)H<sub>2</sub>Se<br>D)H<sub>2</sub>Te
Q53: These questions refer to an atom of
Q64: A magnesium atom is converted to a
Q67: The following figure shows a(a): <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4036/.jpg"
Q78: Which of the following elements will most
Q79: A real gas most closely resembles an
Q88: Write orbital diagrams for the following elements
Q92: What is the molar mass of a