Consider the single-displacement reaction in which zinc metal displaces hydrogen from hydrochloric acid.
A.Write a complete and balanced chemical equation for this reaction.
B.If 3.4
 1023 zinc atoms react with 4.5
1023 zinc atoms react with 4.5
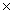 1023 molecules of hydrochloric acid,calculate the mass of zinc chloride produced in this reaction.
1023 molecules of hydrochloric acid,calculate the mass of zinc chloride produced in this reaction.
C.Calculate the number of moles of the excess reactant that remain once the reaction stops.
Definitions:
Ending Inventory
The value of goods available for sale at the end of an accounting period.
Variable Overhead Cost
Overhead costs that vary with the level of production or business activity, such as utilities or materials used in production.
Absorption Costing
A method of cost accounting that includes all manufacturing costs - direct materials, direct labor, and both variable and fixed manufacturing overhead - in the cost of a product.
Variable Costing
A costing method where only variable manufacturing costs are included in the cost of goods sold, with fixed manufacturing overhead treated as a period expense.
Q17: How many orbitals are contained in the
Q22: Nitrogen gas to water
Q38: What is the volume of 1.40 moles
Q42: The percent yield of a reaction can
Q47: The addition of a crystal of sodium
Q60: The formula for the hydrogen carbonate anion
Q61: For any substance,which phase of matter contains
Q70: The amount of energy that must be
Q89: How many moles of hydrogen are
Q103: When a metal reacts with hydrochloric acid<br>A)a