In order to decrease the freezing point of 500.g of water to 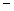 1.0°C,how many grams of ethylene glycol (C2H6O2) must be added? (Kf = 1.86
1.0°C,how many grams of ethylene glycol (C2H6O2) must be added? (Kf = 1.86 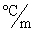 )
)
Definitions:
E-Mail Marketing Spam
Unsolicited and often irrelevant messages sent over email, usually for the purpose of advertising, phishing, or spreading malware.
Opt-In
A policy where individuals choose to receive communications, such as newsletters or marketing emails, by explicitly giving permission.
Privacy Policy
A document that outlines how an organization collects, uses, and protects the personal information of its customers or clients.
Customer Advocacy
A marketing strategy focusing on cultivating strong customer relationships by addressing their needs and concerns, encouraging loyalty and positive word-of-mouth.
Q8: What is the oxidation number of bromine
Q13: When 5.00 g of Al<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub> <sub> </sub><br><img
Q19: Explain how and why the following properties
Q25: How many valence electrons are present in
Q69: What is the maximum number of electrons
Q71: Reduction is the<br>A)gain of protons.<br>B)loss of protons.<br>C)gain
Q74: Which K<sub>eq</sub> value indicates the greatest concentration
Q90: A solution is a<br>A)homogeneous compound.<br>B)heterogeneous compound.<br>C)homogeneous mixture.<br>D)heterogeneous
Q95: The half-life of Sn-110 is 4 hours.If
Q95: The electron configuration of an atom is