In order to decrease the freezing point of 500.g of water to 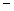 1.0°C,how many grams of ethylene glycol (C2H6O2) must be added? (Kf = 1.86
1.0°C,how many grams of ethylene glycol (C2H6O2) must be added? (Kf = 1.86 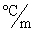 )
)
Definitions:
Binary Large Objects
Known as BLOBs, they are collections of binary data stored as a single entity in a database management system.
Access Database
A Microsoft software that combines a graphical user interface and software-development tools with the relational Microsoft Jet Database Engine.
Field Size
Defines the maximum number of characters that a field can hold in a database.
Caption
Text displayed on screen to provide additional or interpretive information, often used in videos for dialogue or to describe sounds.
Q14: What are the spectator ions when hydrochloric
Q25: What is the concentration of an HCl
Q32: At which temperature would CO<sub>2</sub> gas be
Q35: In aqueous solution the [H<sup>+1</sup>] is 1.4
Q54: Which is the correct reduction half-reaction
Q62: In a basic solution the<br>A)concentration of hydronium
Q66: Which is unsaturated?<br>A)C<sub>3</sub>H<sub>8</sub><br>B)C<sub>4</sub>H<sub>10</sub><br>C)CH<sub>4</sub><br>D)C<sub>5</sub>H<sub>8</sub>
Q71: In which direction will the point of
Q88: Which anion will not precipitate silver ion?<br>A)Chloride<br>B)Nitrate<br>C)Bromide<br>D)Carbonate
Q92: Answer the following questions based on the