What is the pH of a solution whose H3O +1 concentration is 1 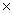 10 -9?
10 -9?
Definitions:
Competitiveness
The ability of a company, industry, or nation to produce goods and services at a lower cost, with higher quality, or in a more desirable manner than competitors.
External Adjustments
Changes made outside of an organization or economy to align with changes in the external environment, such as market demand or regulatory shifts.
Monetary Policy
A central bank’s changing of the money supply to influence interest rates and assist the economy in achieving price-level stability, full employment, and economic growth.
Trade Deficit
Occurs when a country's imports exceed its exports, leading to a negative balance of trade.
Q9: Which type of chemical bond involves the
Q42: A pressure of 340.mm Hg is equal
Q55: The number of possible isomers of C<sub>5</sub>H<sub>12</sub>
Q70: A Th-232 nucleus decays to Pb-208 by
Q74: Which K<sub>eq</sub> value indicates the greatest concentration
Q75: Atoms of the metallic elements generally form
Q83: What is the net ionic equation
Q89: Potassium-42 undergoes beta decay with a half-life
Q92: The vapor pressure of a liquid is
Q98: In the following equilibrium;as the pressure is