In which direction will the point of equilibrium shift when the pressure is decreased in the following equilibrium?
H2 (g) + Cl2 (g) 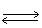 2 HCl (g)
2 HCl (g)
Definitions:
Q3: What is the name for the following
Q4: Which acid and base will combine to
Q24: Which hydrocarbon can undergo a substitution reaction?<br>A)C<sub>2</sub>H<sub>2</sub><br>B)C<sub>6</sub>H<sub>6</sub><br>C)C<sub>3</sub>H<sub>6</sub><br>D)C<sub>4</sub>H<sub>6</sub>
Q35: In aqueous solution the [H<sup>+1</sup>] is 1.4
Q41: During a redox reaction,the oxidation number of
Q46: Bronsted and Lowry defined an acid as
Q52: The K<sub>sp</sub> value for copper(II)sulfide is 8.5
Q93: CH<sub>3</sub>CH=CHCH<sub>2</sub>CH<sub>3</sub> is<br>A)pentane.<br>B)pentyne.<br>C)2-pentene.<br>D)3-pentene.
Q95: Which is a carboxylic acid?<br>A)CH<sub>3</sub>OH<br>B)HCOOH<br>C)CH<sub>3</sub>OCH<sub>3</sub><br>D)CH<sub>4</sub>
Q100: Using Lewis electron dot symbols,show how magnesium