Calculate the pH of a 0.250 M aqueous solution of HNO2.The acid dissociation constant,Ka,for HNO2 is 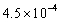 .
.
Definitions:
Mortgage-Backed Securities
Financial instruments that are secured by mortgages, with payments derived from the underlying loans.
Economic Efficiency
The optimal allocation of resources to maximize the production of goods and services without wasting any resources.
Competitive Forces
Pressures or influences that affect an organization's ability to compete in a market, including rivalry among existing competitors, the threat of new entrants, and bargaining power of customers and suppliers.
Market Allocation
A method of distributing goods and services among buyers and sellers wherein prices are determined by supply and demand forces in the market.
Q14: Carbon tetrachloride is a hydrocarbon.
Q18: A sample of liquid water at 100.<sup>
Q30: Which pH is most alkaline?<br>A)1<br>B)5<br>C)7<br>D)12
Q31: Cellulose is a(n)<br>A)ester.<br>B)ether.<br>C)hydrocarbon.<br>D)carbohydrate.
Q32: A U-238 nucleus decays to Pb-206 by
Q33: Which is an ester?<br>A)CH<sub>3</sub>OH<br>B)CH<sub>3</sub>CH<sub>2</sub>COOCH<sub>3</sub><br>C)HCOOH<br>D)HCHO
Q39: What types of molecules are combined to
Q40: A sample of gas is in a
Q49: A key feature of the scientific method
Q58: An ethylene glycol,C<sub>2</sub>H<sub>4</sub>(OH)<sub>2</sub>,solution contains 500.g of ethylene