In the following equilibrium,as temperature increases,the point of equilibrium will CO2 (g) + 2 H2O (g) + 890 kJ 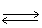 CH4 (g) + 2 O2 (g)
CH4 (g) + 2 O2 (g)
Definitions:
Reimbursement
A repayment for money spent or losses incurred, often from one party to another as compensation.
Independent Contractor
An individual or entity contracted to perform work for another entity as a non-employee, responsible for their own tax obligations and not subject to the hiring entity’s control over how the work is performed.
Contract Authorization
The process or act of giving official or legal approval to a contract, enabling it to be valid and enforceable.
Automatically Terminated
Describes a legal status or condition that ends without the need for any action by a party due to pre-set conditions being met.
Q8: How many pairs of electrons are shared
Q19: CH<sub>3</sub>CH<sub>2</sub>CH(CH<sub>3</sub>)<sub>2</sub> is<br>A)1,1-dimethylpropane<br>B)3,3-dimethylpropane<br>C)2-methylbutane<br>D)4-methylbutane
Q24: Which hydrocarbon can undergo a substitution reaction?<br>A)C<sub>2</sub>H<sub>2</sub><br>B)C<sub>6</sub>H<sub>6</sub><br>C)C<sub>3</sub>H<sub>6</sub><br>D)C<sub>4</sub>H<sub>6</sub>
Q25: The following structure corresponds to <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4036/.jpg"
Q27: If HCl (aq)is added to a saturated
Q36: The statement,"An atom consists of a dense
Q48: The nucleus of U-238 has a mass
Q81: Why do beta particles undergo much larger
Q83: Which compound will form an aqueous solution
Q101: What is the number of valence electrons