True/False
If the concentration of hydronium ions in a solution is 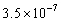 ,the solution is basic.
,the solution is basic.
Definitions:
Related Questions
Q39: In the reaction,2 F<sub>2</sub> + O<sub>2</sub>
Q50: The main reason for the increase in
Q53: U-232 represents a uranium isotope with 92
Q57: What is the molar mass of a
Q61: The change in oxidation number of an
Q74: Which bond type is weakest?<br>A)Polar covalent bond<br>B)Nonpolar
Q80: As the pressure of a sample of
Q80: The end products of the saponification of
Q85: A fatty acid which contains many double
Q97: How many moles of water will be